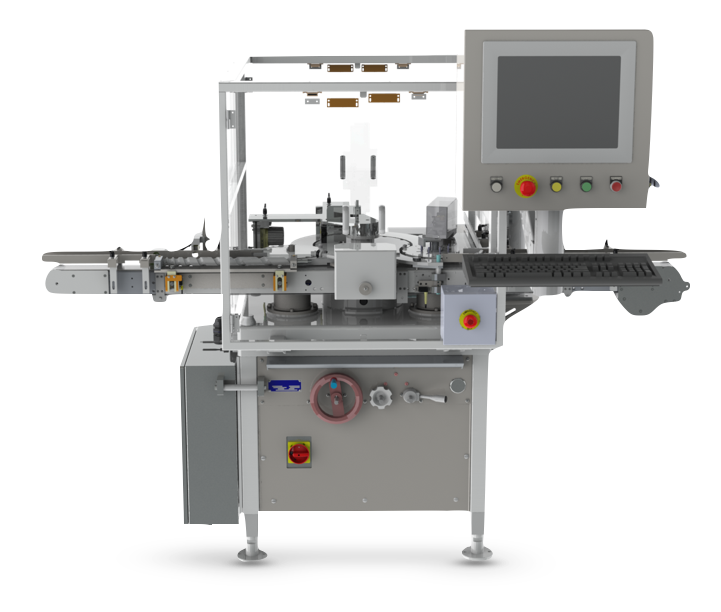
The Details
The VIP has a main star wheel and a discharge star wheel so that non conforming product can be rejected without human intervention. When placed in front of the existing capping equipment, it can be used to test for single or multiple attributes such as raised stopper detection, head space analysis or the presence of moisture using near IR technology. When integrated after the capping process, the Genesis VIP can be used to apply and verify lot numbers or other identifying features or could again be used as a platform for testing analysis. The VIP is designed to easily integrate with all forms of Westcappers® as well as most other commonly used capping machines.
Meeting Annex One Compliance
Annex One: Manufacture of Sterile Medicinal Products
The manufacture of sterile medicinal products covers a wide range of product types, (sterile active substance through to finished dosage form), batch sizes (single unit to multiple units), processes (from highly automated systems to manual processes), primary packaging materials and technologies (e.g. biotechnology, classical small molecule manufacturing and closed systems). This Annex provides general guidance that should be used for all sterile medicinal products and sterile active substances, via adaption, using the principles of Quality Risk Management (QRM), to ensure that microbial, particulate and pyrogen contamination associated with microbes is prevented in the final product.
How the VIP Can Help
Section 121
The Guidance
"Vial capping can be undertaken as an aseptic process using sterilized caps or as a clean process outside the aseptic core. Where this latter approach is adopted, vials should be protected by grade A conditions up to the point of leaving the aseptic processing area, and thereafter stoppered vials should be protected with a grade A air supply until the cap has been crimped."
The VIP Solution
The Genesis VIP can be added to an existing line just prior to the capping operation and use a two camera system to measure 100% of the vials against a pre-determined raised stopper height standard. In this case each measurement is taken by determining the distance between the top surface of the vial and the bottom surface of the stopper. Once again, non conforming vials are rejected, prior to capping without human intervention.
Section 123
The Guidance
"Containers sealed under vacuum should be tested for maintenance of that vacuum after an appropriate, pre-determined period."
The VIP Solution
Through the use of the VIP vials can be inspected at a later time by mounting a laser headspace analysis system such as produced by Lighthouse Instruments to determine if a vial has indeed held vacuum.