Vera
Residual Seal Force Tester
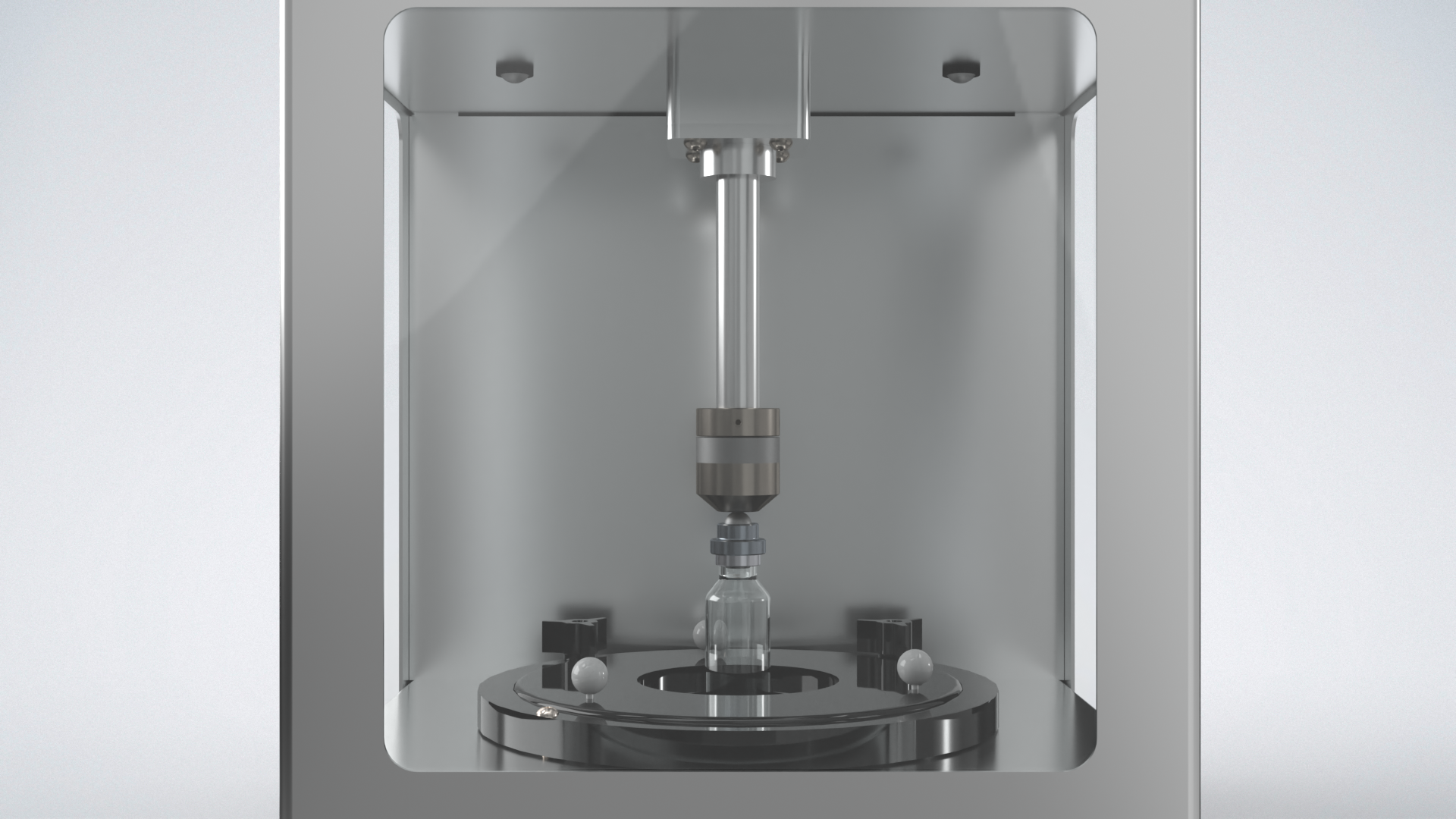
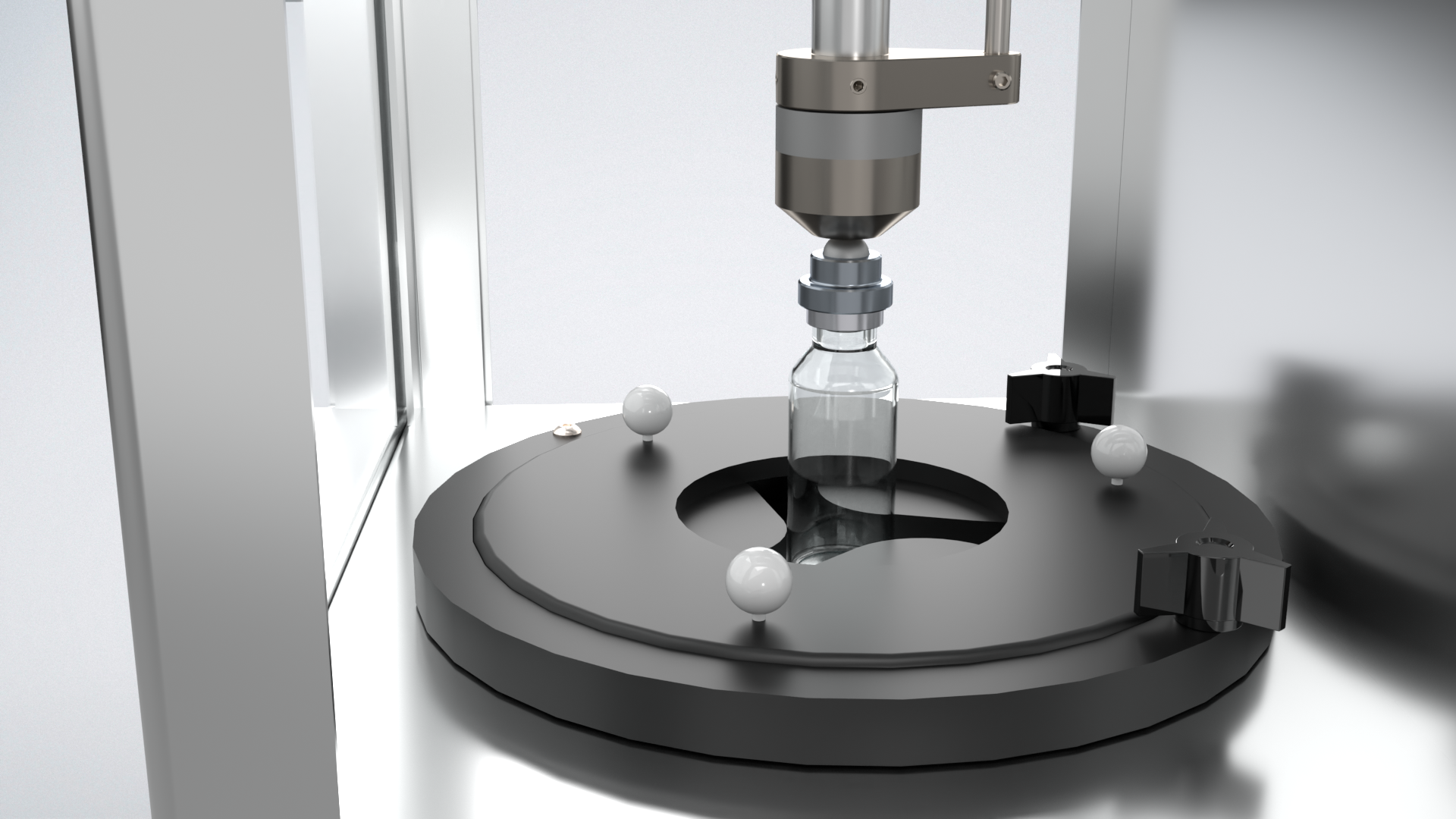
The Vera® Residual Seal Force Tester provides a fast, reliable way to verify vial seal tightness, helping you optimize capping setup and maintain container-closure integrity throughout your process.

Gallery
![]()
Ensure Container‑Closure Integrity with Precision
The Vera has been engineered to assess the critical seal‐tightness of parenteral vials by measuring Residual Seal Force (RSF) — the force that the elastomeric stopper flange continues to exert on the vial land after the aluminium cap has been crimped. RSF is explicitly recognised in USP <1207> as a seal quality attribute, and multiple studies have demonstrated a clear correlation between RSF and container closure integrity (CCI) performance. By quantifying that “spring‐force” stored in the compressed stopper and tracking its decay over time (via a Maxwell/Maxwell‐Wiechert model), the Vera enables the establishment of robust capping parameters (stopper compression %, crimp height) and the monitoring of manufacturing process control. In cold-chain, biologic and other mission-critical parenterals, maintaining sufficient RSF is essential to avoid seal degradation over shelf life or shipping. Therefore, by integrating Vera RSF testing into development, validation, and routine production oversight, you achieve a data-driven seal quality assurance program that aligns with FDA expectations for lifecycle packaging integrity, supports risk management, and enhances patient safety.
Why RSF Is Critically Important for Parenteral Vial Integrity
-
Sterility and Patient Safety
If the seal is compromised (even by micro-gaps), ingress of microorganisms, moisture, or gases (e.g., oxygen) may occur; in worst-case scenarios this threatens sterility, degrades the drug, or causes patient harm. Maintaining adequate Residual Seal Force (RSF) at initial set-up and over time helps prevent such ingress.
-
Shelf Life and Cold/Harsh Conditions
Many parenterals—especially biologics, cell therapies, and vaccines—require cold-chain storage and transport extremes. Elastomer behavior under those conditions (time-temperature superposition, stress relaxation) shrinks the margin between a sealed and a failed system. RSF testing quantifies that margin and verifies the system remains sealed under worst-case conditions.
-
Process Repeatability & Regulatory Compliance
Capping is high-throughput, and normal variation—stopper thickness tolerance, cap material variation, crimp head alignment, torque, etc.—can lead to under-compression (insufficient RSF) or over-compression (risk of glass damage or cap deformation). Monitoring RSF effectively monitors the combined outcome of these interacting variables. Process set-up must be controlled to maintain RSF and avoid failures.
-
Correlation to Leakage Risk & Life-Cycle Monitoring
As shown in key reviews, the higher the stopper flange compression percentage, the higher the RSF value—and the lower the leak rate. In practice, RSF becomes a sentinel metric for leak risk. Once correlation is established for your system, RSF results can be used as a screening/predictive tool rather than relying solely on slower leak-test methods—an advantage for production control.
The Genesis Vera® is more than a testing device — it’s a practical tool for building confidence in your container closure system. By incorporating RSF measurements into your development, validation, and production routines, you can create a data-driven seal integrity program that enhances quality, repeatability, and compliance.
-
Supporting Development and Validation
During container closure development, the Vera can be used to establish baseline RSF values for each vial, stopper, and cap combination. Measure RSF immediately after sealing (t = 0), then again at intervals such as 1 hour, 24 hours, and 7 days to track the natural relaxation of the elastomer. This creates an “RSF life-curve” and helps define the minimum acceptable RSF for your system.
-
Establishing Process Limits
Once target RSF levels are known the Vera can be used to verify that production lots meet those limits. If results fall below threshold, the data points directly to potential root causes — such as stopper variation, cap misalignment, or capping machine setup — allowing rapid corrective action.
-
Correlating RSF to Seal Integrity
In validation studies, RSF measurements can be run in parallel with leak tests such as helium or vacuum decay. This demonstrates how RSF values correlate to actual container closure integrity (CCI) performance. Once correlation is established, RSF can be confidently used as a predictive seal-quality metric — saving time while maintaining compliance.
-
Ongoing Line Monitoring and Control
In routine production, RSF testing with the Vera provides continuous visibility into the health of your capping process. Periodic sampling of finished or test vials can reveal if machine settings have drifted, if components have changed, or if environmental factors (like cold chain exposure) are affecting seal performance. The Vera becomes a proactive part of your quality control program — not just a validation tool.
-
Regulatory Confidence and Documentation
RSF testing is increasingly recognized by regulatory authorities as part of a comprehensive container closure integrity strategy. Including Vera test data in your validation reports or submissions demonstrates a science-based approach to capping process control. This aligns with FDA expectations that packaging integrity be supported by quantifiable, data-driven evidence.
The FDA recognizes the use of RSF as a predictor of seal integrity.
The FDA in its Guidance for Industry Container and Closure System Integrity Testing in Lieu of Sterility Testing as a Component of the Stability Protocol for Sterile Products recognizes the usefulness of properly validated seal force testing. Most importantly, RSF is useful in the establishment, validation and control of capping machine settings.
Key Features
![]()
Modern System Controls
The control platform utilizes a reliable PLC and modern PC architecture for all data acquisition and resultant processing.
![]()
Modern Touch Screen Interface
The Vera features a high resolution 10.1 inch touchscreen.
![]()
Adjustable Vial Rest
The vial rest is adjustable to fit any vial size from 8mm to 88mm without having to change parts.
![]()
Dual SI & Imperial Units
Units for reporting can be switched between Imperial (Lbf Inches) and (Newtons mm). The selection becomes the default for future operation.
![]()
Graphical Data Display
The 10.1 inch screen allows for rich graphical data and reports to be displayed right on the tester.
![]()
Customizable Sample Labeling
The ability to add custom labels to your samples allows for more robust sample tracking.
![]()
Improved Accuracy
The Vera obtains several hundred force data points to allow for a more accurate analysis.
![]()
Improved Force Calibration
The force feedback entails a step by step process with a three point final verification and date/time stamp.
![]()
New Distance Calibration
A distance calibration process has been added and includes a three point final verification with date/time stamp.
![]()
Data Export
The ability to export reports and graphs to a Microsoft Excel spreadsheet or to Crystal Reports. A licensed copy of Excel is included with the unit.
![]()
Wireless Mouse & Keyboard Support
Wireless mouse and keyboard support is built in to the embedded PC.
![]()
User Security Management
Manage all users with fine grain security controls to restrict access and control use cases. Options are available to automatically obtain user credentials from a customer's secure server.
![]()
21 CFR Part 11 Capable
The Vera is capable to be 21 CFR part 11 compliant.
![]()
VHP Sterilization
The Vera has been certified for use with Vaporized Hydrogen Peroxide.
![]()
CE Compliant
The Vera has been certified to be CE compliant.
Operation & Vial Size Limits
- Measures residual seal force (RSF) for parenteral vials sealed with stoppers, lined seals, and aluminum caps.
- Supports aluminum caps with or without plastic buttons (button removal recommended for testing).
- Accommodates vial diameters from 8 mm to 88 mm and heights up to 6.47 inches.
- Compatible with a wide range of cap sizes, including 7.5, 8, 10, 11, 13, 16.5, 17, 18, 20, 28, 30, 32, and 43 mm.
- Measures residual seal forces from 3 lbf to 35 lbf with high repeatability.
- Specialty cap materials and styles available upon engineering evaluation.
Hardware Specifications
Impact of Vial Capping on Residual Seal Force and Container Closure Integrity | PDA Journal
The vial capping process is a critical unit operation during drug product manufacturing, as it could possibly generate cosmetic defects or even affect container closure integrity. Yet there is significant variability in capping equipment and processes, and their relation to potential defects or container closure integrity has not been thoroughly studied. In this study we applied several methods—residual seal force tester, a self-developed system of a piezo force sensor measurement, and computed tomography—to characterize different container closure system combinations that had been sealed using different capping process parameter settings.
Roman Mathaes, Hanns-Christian Mahler, Yves Roggo, Robert Ovadia, Philippe Lam, Oliver Stauch, Martin Vogt, Holger Roehl, Joerg Huwyler, Silke Mohl and Alexander Streubel