Annex One: Manufacture of Sterile Medicinal Products
The manufacture of sterile medicinal products covers a wide range of product types, (sterile active substance through to finished dosage form), batch sizes (single unit to multiple units), processes (from highly automated systems to manual processes), primary packaging materials and technologies (e.g. biotechnology, classical small molecule manufacturing and closed systems). This Annex provides general guidance that should be used for all sterile medicinal products and sterile active substances, via adaption, using the principles of Quality Risk Management (QRM), to ensure that microbial, particulate and pyrogen contamination associated with microbes is prevented in the final product.
![]()
How We Help You Meet the Guidance...
Section 119
The Guidance
"As the equipment used to crimp vial caps can generate large quantities of non-viable particulates, the equipment should be located at a separate station equipped with adequate air extraction".
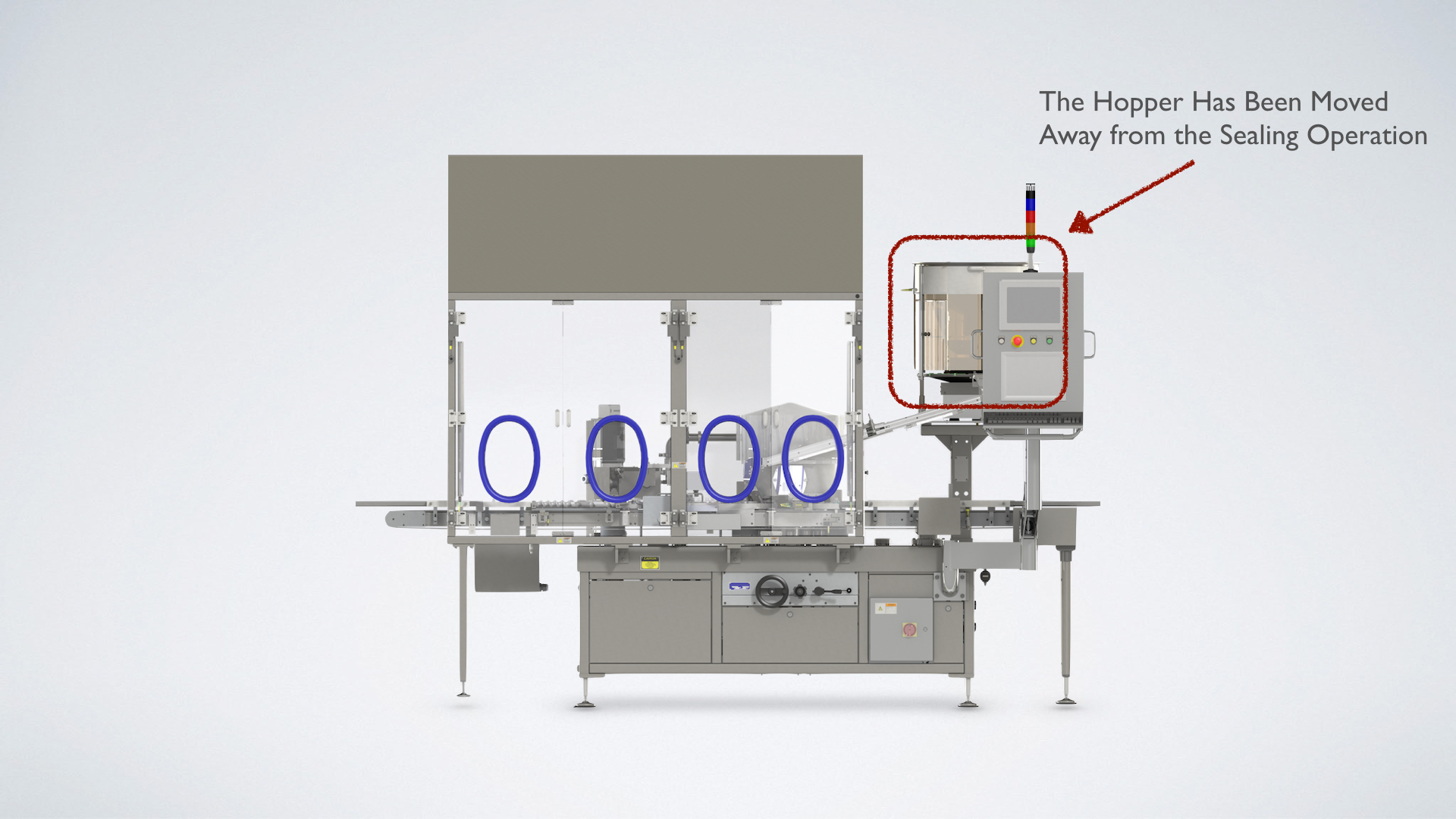
Our Solution
One of the greatest sources of particulate generation in the sealing operation occurs in the hopping of the aluminum seals. Genesis has moved the hopper of the Westcapper® to the discharge side of the machine separated from the capping station by a partition. The hopper is uniquely designed as a first in/first out unit where the aluminum seals are turned as soon as possible so that the cut edge of the aluminum seal is not able to abrade against other seals. The hopper also electronically monitors the flow of the aluminum seals so that it stops all rotation and vibration when additional flow is not needed. This also decreases particulate generation. One of the newest developments at Genesis is an air extraction system mounted behind the sealing rail. This system is designed to remove particulates created as the sealing rail folds the aluminum seal.
Section 120
The Guidance
"Vial capping can be undertaken as an aseptic process using sterilized caps or as a clean process outside the aseptic core. Where this latter approach is adopted, vials should be protected by grade A conditions up to the point of leaving the aseptic processing area, and thereafter stoppered vials should be protected with a grade A air supply until the cap has been crimped."
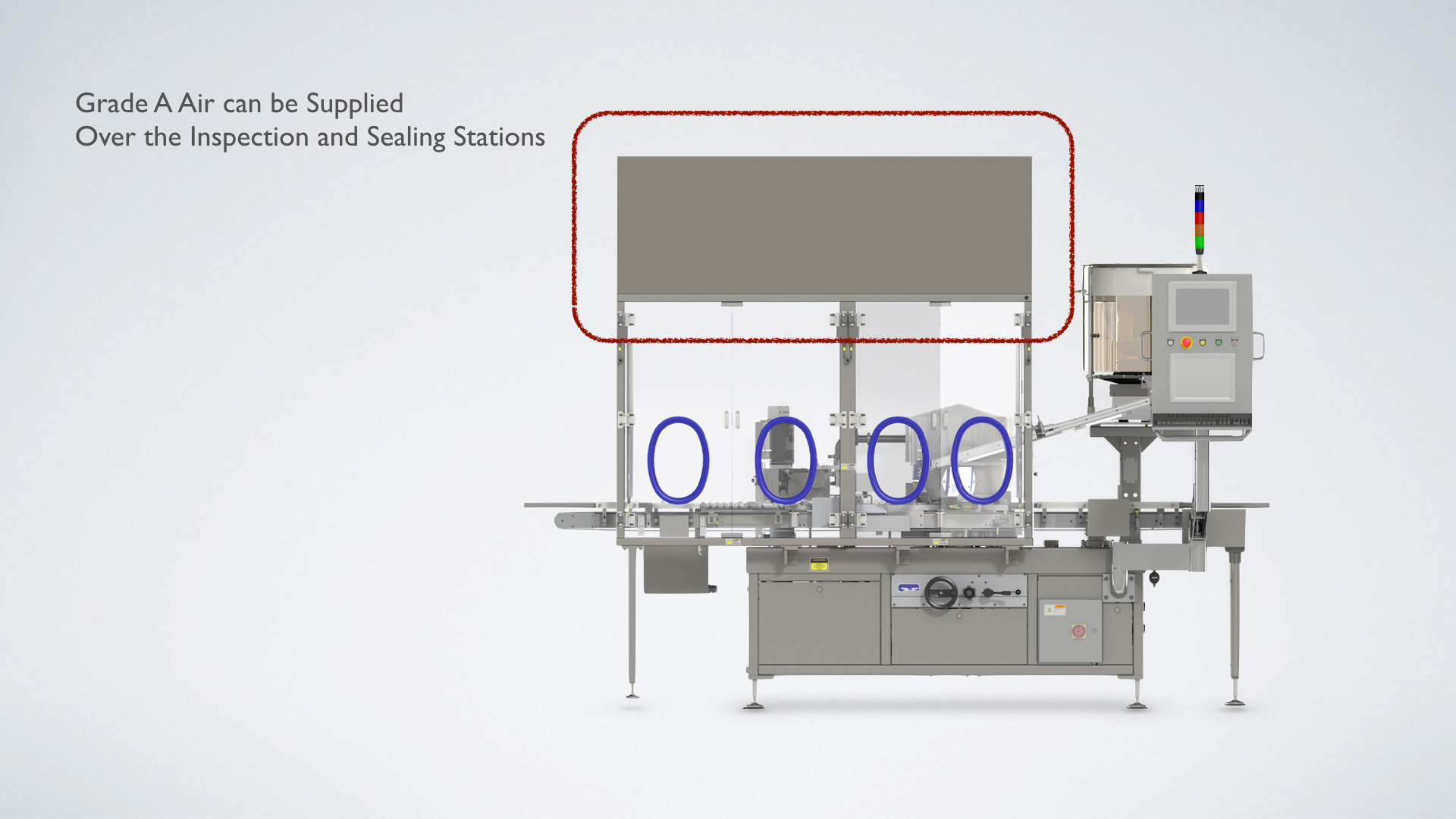
Our Solution
When using non sterilized aluminum seals, grade A air may be supplied over the raised stopper and sealing platforms. When using pre-sterilized aluminum seals, grade A air may be supplied over the hopper as well as the first two stations.
Section 121
The Guidance
"Vials with missing or displaced stoppers should be rejected prior to capping. Where human intervention is required at the capping station, appropriate technology should be used to prevent direct contact with the vials and to minimize microbial contamination."
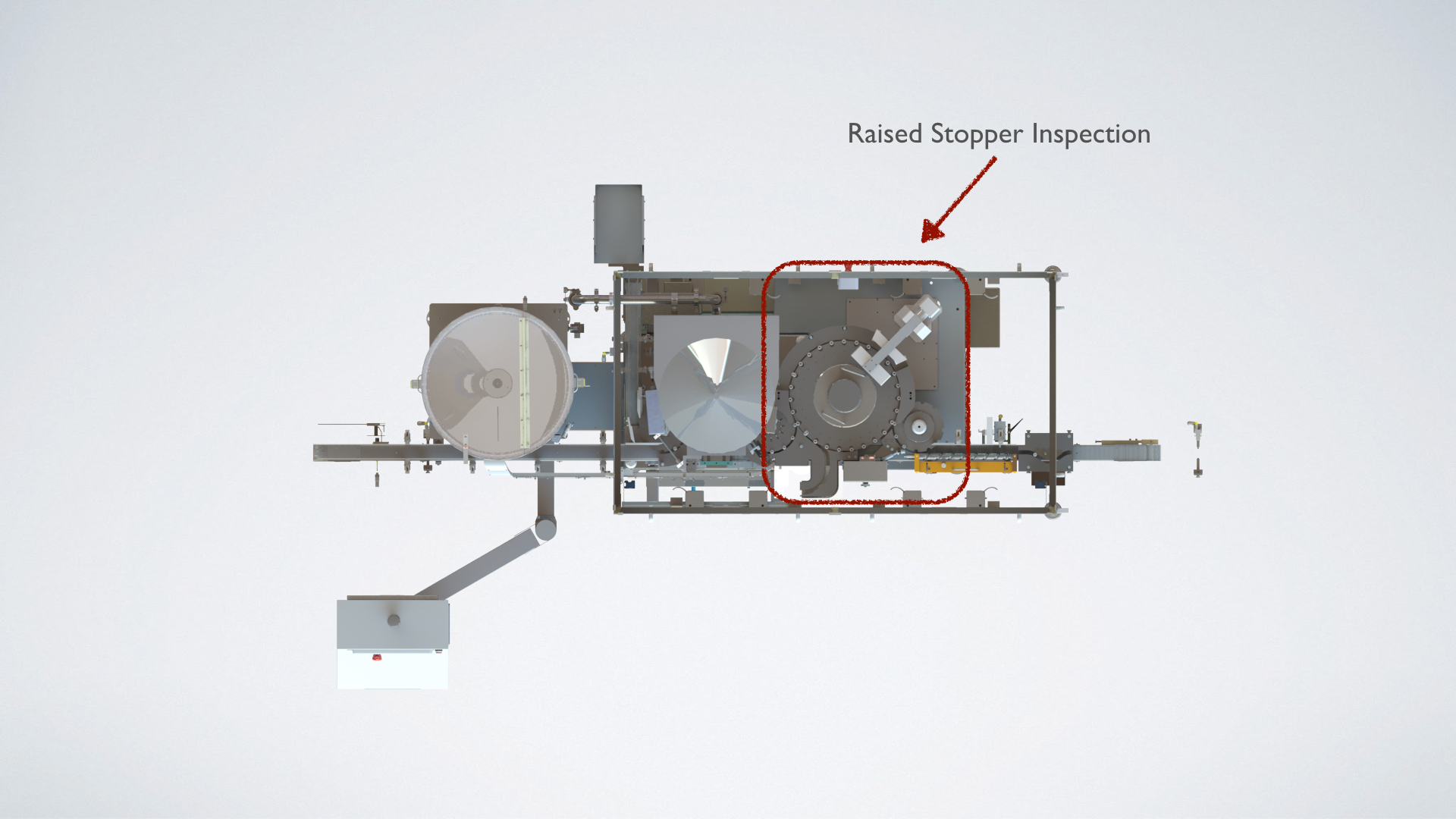
Our Solution
Vials are inspected for the presence of a stopper as well as for raised or tilted stoppers prior to the sealing station. Defective vials are automatically rejected without the need for human intervention.
Section 122
The Guidance
"Restricted access barriers and isolators may be beneficial in assuring the required conditions and minimizing direct human interventions into the capping operation."
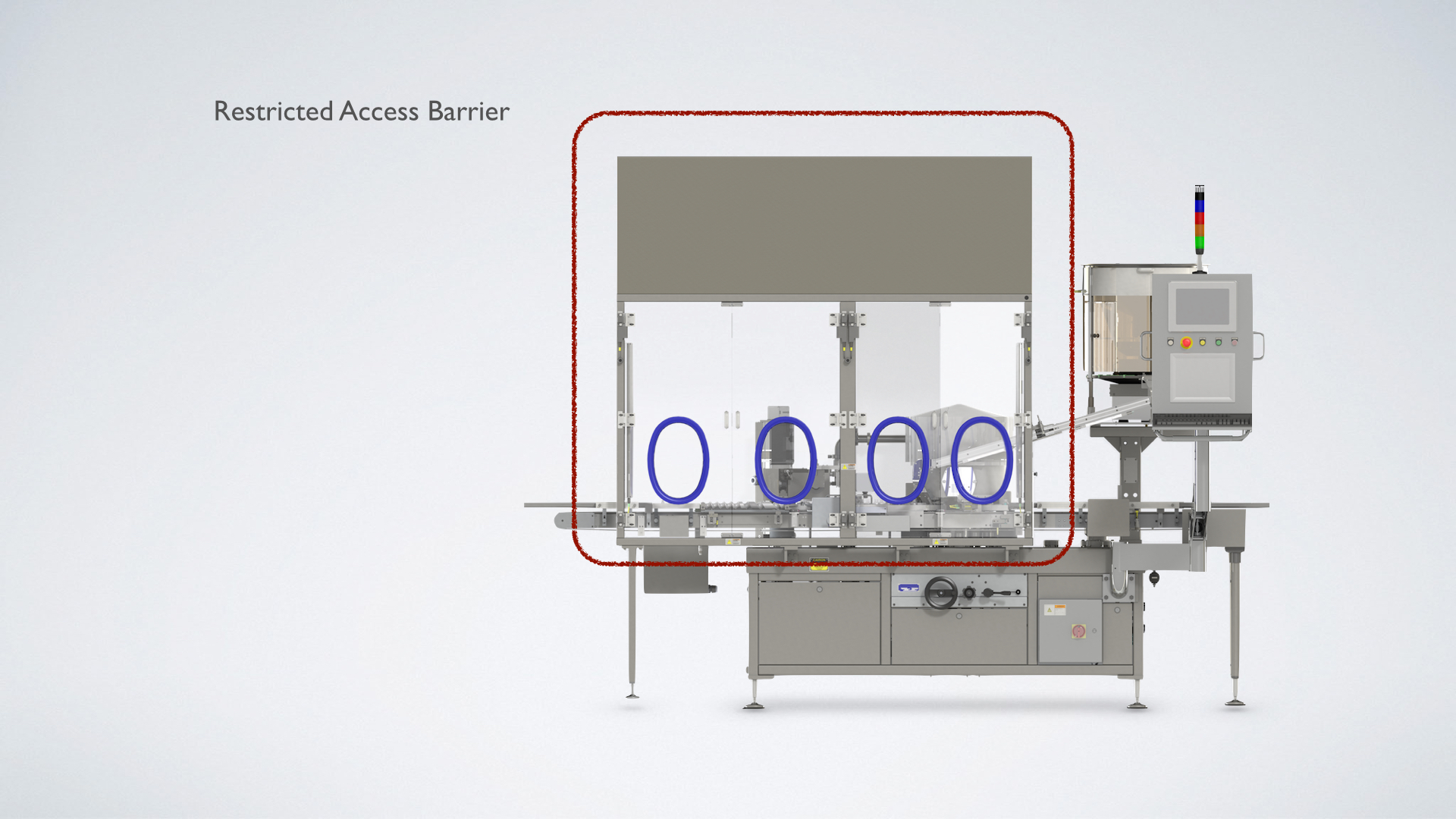
Our Solution
Westcappers® are available with customizable safety enclosures which help isolate the sealing process. They are also available with air monitoring systems as well as viable and nonviable particulate counters that trigger alarm systems. Glove ports along with safety light curtains further reduce direct human intervention.