
The Leader in Seal Integrity
Genesis Packaging Technologies is a worldwide leader in the science and technology of Parenteral Vial Sealing and Residual Seal Force testing. We provide advanced vial sealing equipment for the packaging of critical injectable pharmaceutical products. Offering our customers the tools and knowledge to consistently achieve Container Closure Integrity remains our priority.

PDA Parenteral Packaging Conference
April 14-15, 2026 | Munich Marriott City West | Munich, Germany
Join us in Munich this April for the PDA Parenteral Packaging Conference, where experts and innovators will come together to explore the future of packaging systems that protect patients and ensure the highest standards of product quality. Over the past year, parenteral packaging has seen significant advancements alongside new complexities — from evolving regulatory expectations to breakthroughs in material development and the growing role of digital tools. This conference provides a unique forum to navigate these changes, share knowledge, and contribute to the continued evolution of packaging science. Stop by Table 30.
Engineering Better Performance Together
Genesis | Mitsubishi Electric
See how Genesis partnered with Mitsubishi Electric to enhance our pharmaceutical sealing equipment, transitioning from a customized motion platform to a streamlined servo-driven system that delivers shorter lead times, faster and more consistent builds, and greater value for our customers. Watch the video to learn how this collaboration is driving the next evolution in vial capping performance.
RW Westcapper®
High Speed, Large Volume, Vial Sealing
Ideal for large production runs of time sensitive products such as lyophilized vaccines the Genesis RW Westcapper is capable of sealing parenteral vials at up to 750 vials per minute.
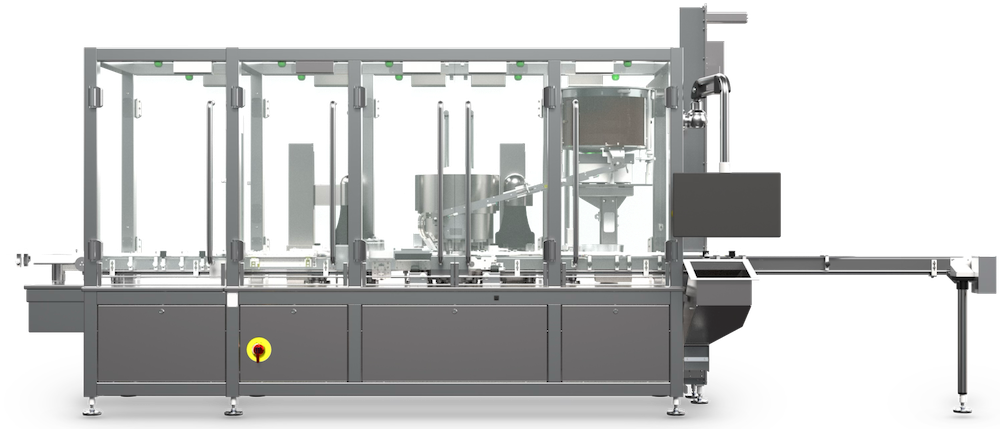

Aptus
Modular Vial Crimping System
The Genesis Aptus is a fully automated, servo driven, constant motion, rotary crimper. The Genesis Aptus is capable of sealing both large and small parenteral vials at up to 300 vials per minute.
Integra
Laboratory Vial Crimper
The Integra Laboratory Crimper is a small vial crimper that is ideal for development, pre-clinical, clinical, pilot and compounding pharmacy operations. Available in four configurations the Integra can meet your small batch size needs assuring the best possible seal integrity.


Vera
Residual Seal Force Tester
The Vera® Residual Seal Force Tester provides a fast, reliable way to verify vial seal tightness by measuring Residual Seal Force (RSF) - the compressive force the stopper continues to exert on the vial land after capping - helping you optimize your capping setup and maintain robust container-closure integrity.
Advisum AI™
Transform Visual Inspection. Reduce Risk. Accelerate ROI.
Through our partnership with Boon Logic, Genesis introduces Advisum - an AI-powered visual inspection platform built on unsupervised machine learning. Advisum adapts faster, detects defects more accurately, and simplifies validation, helping manufacturers achieve measurable improvements and rapid return on investment.
Ready to modernize your inspection process? Discover how Advisum AI can elevate quality, efficiency, and confidence across your packaging operations.
- AI-powered inspection that adapts without complex rule programming
- Improved defect detection and reduced false rejects
- Faster validation and simplified system maintenance
- Lower operational costs and quicker return on investment
- A future-ready solution backed by Genesis’ 75-year legacy in parenteral packaging innovation